Join our subscribers list to get the latest news, updates and special offers delivered directly in your inbox.

We 'll fill and ship your scripts in:
Live prescription fulfillment time
Our goal is to deliver the best services and prices possible. Everyone at Absolute Pharmacy is committed to our patients and practitioners
Absolute Pharmacy covers most of continental United States, Hawaii and Alaska, making it an easy choice for providers. See map for details.
If our state coverage or great shipping options aren't enough, you have more reasons to choose Absolute Pharmacy:

Treating severe PTSD, severe anxiety disorders, severe bipolar disorder, drug addiction rehabilitation, neuropathic pain, CRPS or RSD, cancer pain syndromes, and phantom limb pain may have a new ally.
Testosterone therapy can offer several potential benefits, including improved muscle mass and bone density, increased sex drive, improved mood and energy levels, and improved cognitive function.
Semaglutide works by stimulating the GLP-1 receptors in the body, which results in a variety of metabolic effects and plays a role in regulating appetite and glucose metabolism.
Contact Absolute to see how we can help your practice today.
Keep Compliance In Mind
Absolute Pharmacy prides itself in automatically assisting telehealth prescribers with regulatory compliance driven AI, that automatically determines state licensing requirements, and registrations directly through our eRX portal
Physician state licensing is at the core of
our E-Prescribing software
Did you know some states require additional CS Licensing?
Our system does!
We can compound almost anything, but here are a few of our specialties.






Instant answers are demanded these days, and Absolute Pharmacy has built a platform to get you what you want, when you want it, without delay!
We accomplish this in many ways, allowing you and your staff to concentrate on your patients and running your business, not sending emails or making phone calls.
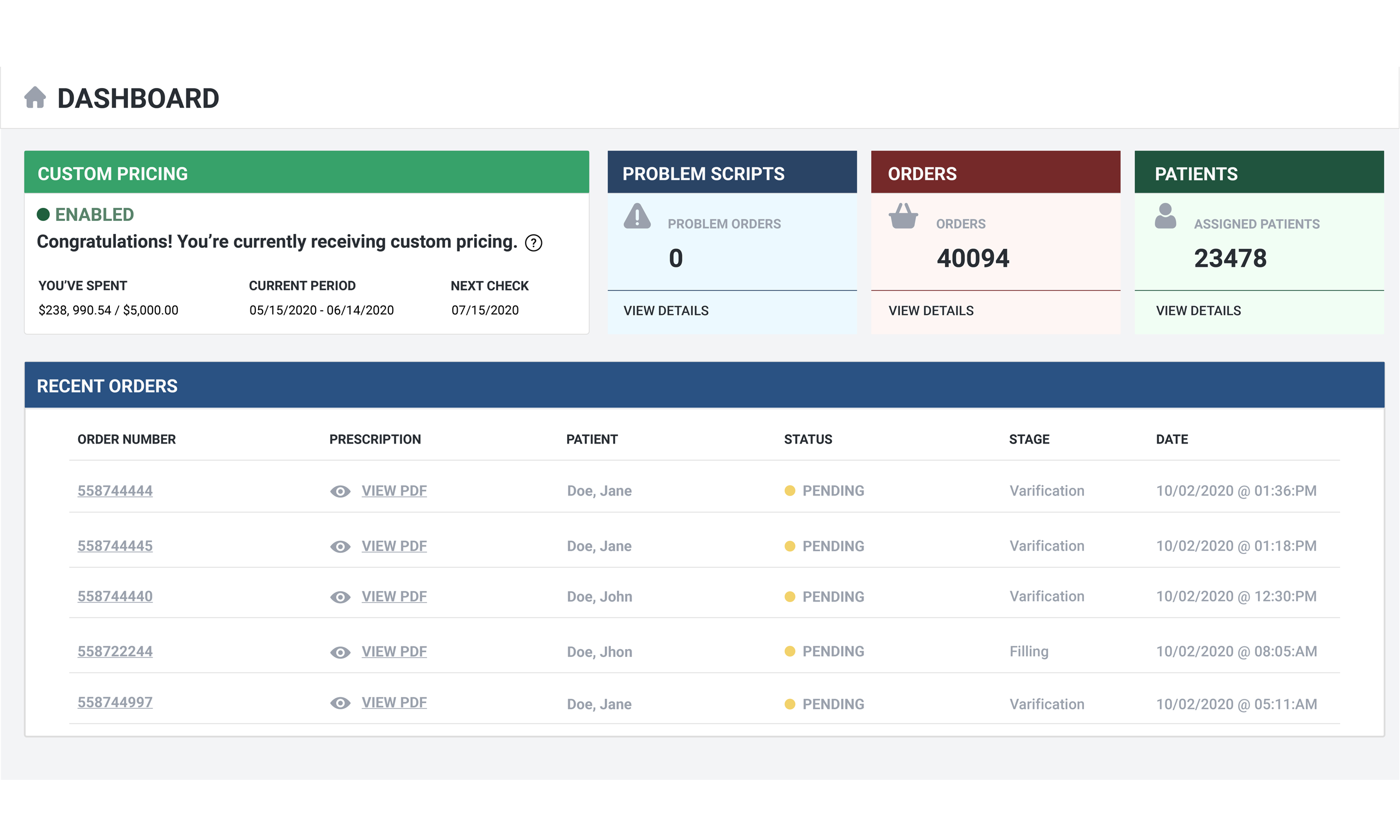
We are changing the way practitioners interact with our pharmacy by using the latest and best
available technologies in our proprietary software. Your time is better spent looking
after your patients - Not calling pharmacies getting status on orders. All of the
information you need will be in your portal, in real time, always up to date.
You can also
integrate with our software via API. Contact us for more information
“The Absolute Portal is a game-changer for us. All of the info we need is available instantly. Everything we need to know is always there.”
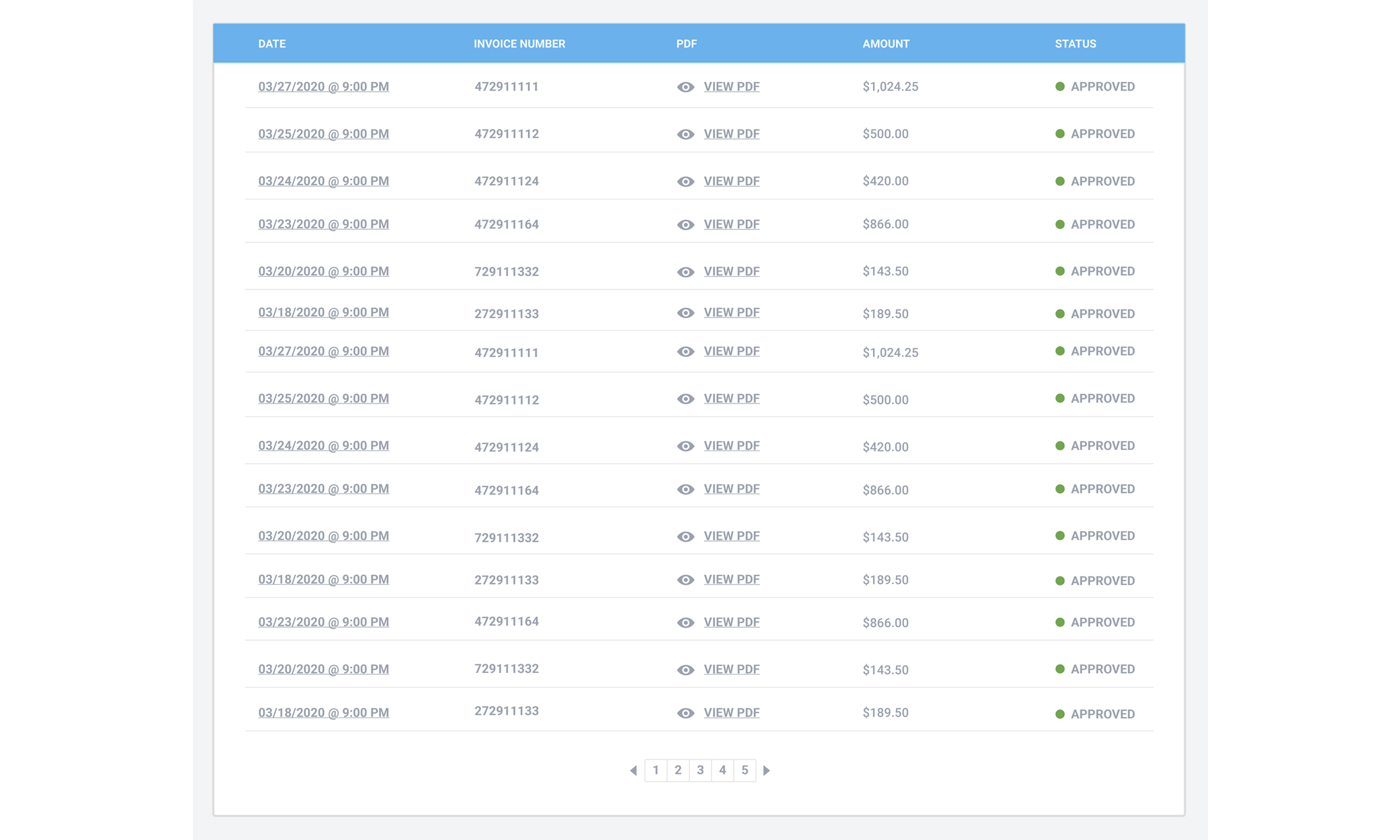
Trust your invoice! We spent a tremendous amount of time developing an accurate but simple automated invoicing system. All of your invoices will aways be at your fingertips from the dashboard with a clear breakdown of all costs. The days of wondering about charges from your pharmacy are OVER!
Our software has so many other great features to offer.
The best way for you to see it all is to create an account and experience it yourself!
All-in-one platform
We didn't have enough space to list it all here. We hope you understand.